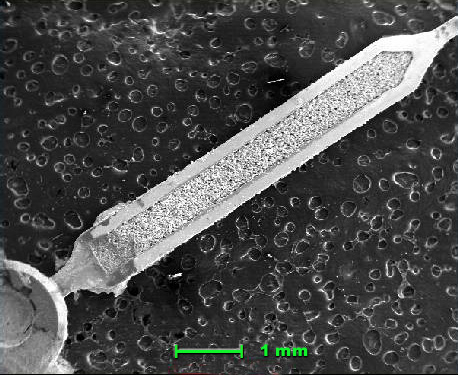
Vintage watch. Hour hand, scanning electron microscope, 20x. Ordinary photograph of the watch is further below.
This watch was coated with an optically invisible thin layer of carbon, to aid in imaging. Note the filler material in the hand. Gamma ray spectroscopy was performed on the material, and showed the presence of radium 226, which has a half life of 1,602 years, and therefore is still, and will continue for a long time to be, radioactive. The way these luminesced is as follows: the radium 226 decays, and emits high-energy beta particles. These will interact with another substance in the dial paint mixture, a scintillator.
This substance will absorb some of the energy of the beta, and when it returns to ground state, it will give up some of that energy in the form of visible light, a "scintillation event." This is the glow we see from the watch. Why then do some of these old watches refuse to glow? The radium is still there and will be for some centuries. It seems that the scintillator material, in most cases Zinc Sulfide-Silver, eventually breaks down, suffers degredation of the crystal structure, and loses it's ability to scintillate. What a shame. See further pages for higher magnification and some surprises....